TUST College of Chemical Engineering (CCE) research team published three papers in Journal of Catalysis (Impact Factor 7.888), one of the most traditional and influential journals in the field of physical chemistry and chemical engineering.
Organic Sulphur-containing compounds in fuel oil will form SOX after combustion, which is a major factor causing air pollution, especially haze and other serious environmental problems. Therefore, deep desulfurization is a long-term and important topic facing clean fuel production. In refinery, the sulfur in distillate oil is mainly removed by hydrodesulphurization (HDS) in hydro refining process. Among them, dibenzothiophene aromatic heterocyclic sulfur compounds are the most difficult sulfur compounds to be removed by HDS reaction. Their reaction networks are complex, containing a variety of sulfur-containing intermediates and different types of C-S bonds, which restricts the understanding of the C-S bond fracture mechanism and the structure-activity relationship of catalysts.
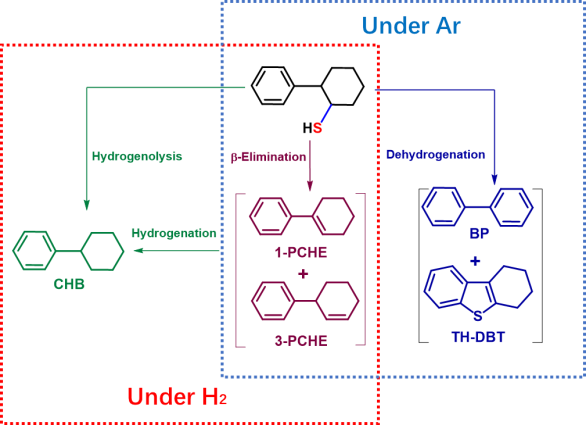
In order to solve this problem, Professor Li Xiang's team designed and synthesized 2-phenylcyclohexanethiol model compounds containing only alkyl C -- S bond, and systematically studied its application in new transition metal phosphates (Journal of Catalysis, 2020, 383, 331-342) and traditional sulfide catalysts (Journal of Catalysis, 2021, Inpress, DOI: 10.1016 / j.j the 2021.01.017331-342) on the desulfurization reaction. Through product analysis and kinetic study, the fracture mode and mechanism of alkyl C - S bond were fully understood and revealed. The β-elimination and hydrogen lysis reactions of naphthenic C-S bonds were identified, and two new reaction pathways of dehydrogen-desulfurization and dehydrogen-cyclamation were also found, the latter of which was difficult to be observed under conventional hydrogen conditions. Meanwhile, a new understanding of the structure-activity relationships of the catalysts was also obtained. These results provide a new idea for revealing the hydro desulfurization reaction mechanism of aromatic heterocyclic Sulphur-containing compounds and constructing efficient hydro desulfurization catalysts.
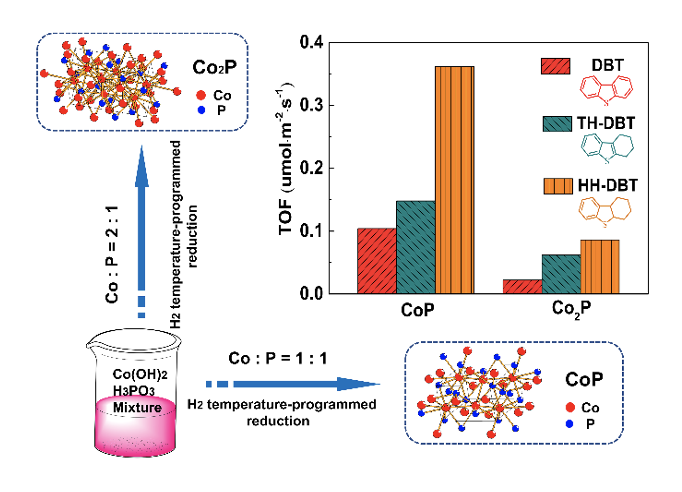
Transition metal phosphates are a new type of deep hydro desulfurization catalysts due to their high activity and high sulfur resistance stability, but their preparation is difficult. The reduction of transition metal phosphate precursors by temperature-programmed reduction in hydrogen atmosphere is the most common method for the preparation of transition metal phosphate. Due to the very strong P -- O bond of phosphate, its reduction can only take place at very high temperatures (BBB 0 500 oC), resulting in phosphorus loss. Therefore, excessive phosphorus should be added in the preparation of transition metal phosphates of group VIII such as Ni2P and COP. This excess phosphorus severely inhibits the activity of the catalyst. Professor Li xiang team to Co (OH) 2 and phosphorus containing low state of H3PO3 mixture replace phosphate as precursors, the hydrogen temperature programmed reduction method is adopted according to the P/Co were prepared to avoid, stoichiometric ratio, roasting Cop and Co2P (Journal of Catalysis, 2021, inpress, DOI: 10.1016 / j.j the 2020.08.030). This method overcomes the difficulty of preparing group VIII transition metal phosphides with constant ratio from conventional hydrogen programmed temperature reduction phosphate precursors, and it is found that COP with stoichiometric P/ metal ratio has high HDS activity.
The full paper link:
https://www.sciencedirect.com/science/article/abs/pii/S0021951720300245
https://www.sciencedirect.com/science/article/abs/pii/S0021951720303523
https://www.sciencedirect.com/science/article/abs/pii/S002195172100018X

Tianjin University of Science & Technology
1038 Dagu Nanlu, Hexi District, Tianjin, P.R.china